
Myrbetriq is a prescription medicine for adults approved by the U.S. Food and Drug Administration (FDA) to treat the overactive bladder symptoms of urgency, frequency, and leakage.
Myrbetriq was shown in studies to be effective in treating OAB symptoms of urgency, frequency and leaks within the first 4 to 8 weeks.
With OAB, the bladder muscle suddenly contracts before the bladder is full. This causes frequent and sudden urges to urinate that may be uncontrollable. These urges can sometimes lead to leakage or accidental wetting.
When taken as directed, Myrbetriq can help relax the smooth muscle that surrounds the bladder and increase the bladder's ability to store urine.
Your doctor may prescribe Myrbetriq if you:
Watch this video to learn more about your bladder and Myrbetriq
Watch an animated explanation of Myrbetriq at work.
Do not take Myrbetriq if you have an allergy to mirabegron or any of the ingredients in Myrbetriq.
Myrbetriq may cause serious side effects including:
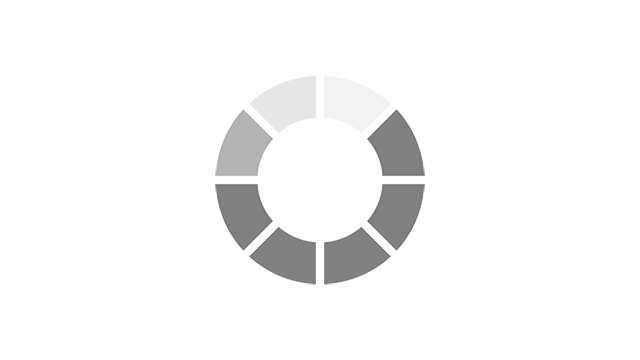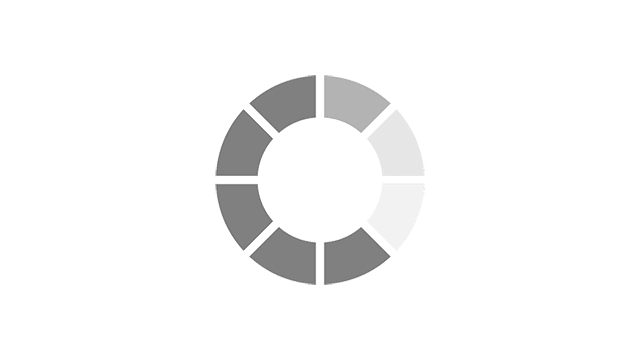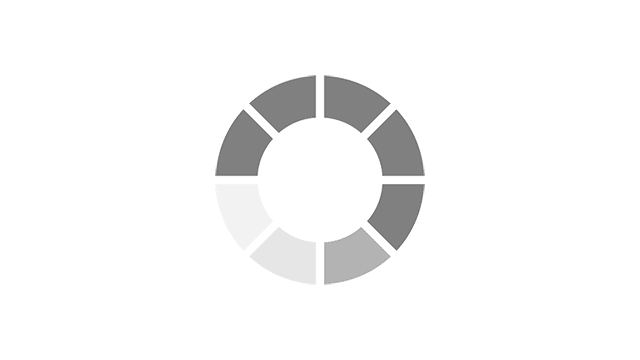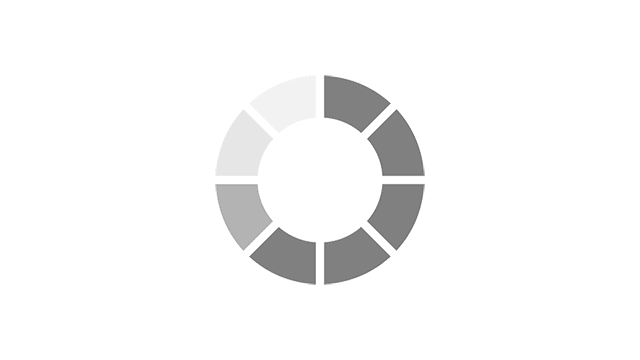
Most common reported side effects of Myrbetriq in a 52-week study of 812 people living with OAB
HOW SIDE EFFECTS OF MYRBETRIQ WERE DETERMINED:
The 52-week Myrbetriq Safety Study Explained:
The study involved 812 people taking Myrbetriq 50 mg 1 time a day for up to 52 weeks. People reported their side effects. Another group of 812 people were given the standard treatment (Active Control), and they reported their side effects.
Most common side effects reported by greater than 3% of people treated with Myrbetriq 50 mg 1 time a day in Study 4.
What is an ‘Active Control’ for clinical studies?
This is the name for the standard treatment people with a certain condition are given.
Click on the side effect(s) to see the percentage of patients who were affected
Myrbetriq 50 mg
9.2%
Active Control
9.6%
Myrbetriq 50 mg
5.9%
Active Control
6.4%
Myrbetriq 50 mg
4.1%
Active Control
2.5%
Myrbetriq 50 mg
3.9%
Active Control
3.1%
Tell your doctor if you have any side effect that bothers you or that does not go away or if you have swelling of the face, lips, tongue, or throat, hives, skin rash or itching while taking Myrbetriq. These are not all the possible side effects of Myrbetriq. For more information, ask your doctor or pharmacist.
Use of Myrbetriq
MYRBETRIQ® (mirabegron extended-release tablets) is a prescription medicine for adults used to treat overactive bladder (OAB) with symptoms of urgency, frequency and leakage.
Do not take MYRBETRIQ if you are allergic to mirabegron or any ingredients in MYRBETRIQ.
MYRBETRIQ may cause your blood pressure to increase or make your blood pressure worse if you have a history of high blood pressure. You and your doctor should check your blood pressure while you are taking MYRBETRIQ. Call your doctor if you have increased blood pressure.
MYRBETRIQ may increase your chances of not being able to empty your bladder. Tell your doctor right away if you have trouble emptying your bladder or you have a weak urine stream. MYRBETRIQ may cause an allergic reaction with swelling of the face, lips, throat or tongue with or without difficulty breathing. Stop using MYRBETRIQ and go to the nearest hospital emergency room right away.
Tell your doctor about all the medicines you take including medications for overactive bladder or other medicines especially thioridazine (Mellaril™ and Mellaril-S™), flecainide (Tambocor®), propafenone (Rythmol®), digoxin (Lanoxin®) or solifenacin succinate (VESIcare®). MYRBETRIQ may affect the way other medicines work, and other medicines may affect how MYRBETRIQ works.
Before taking MYRBETRIQ, tell your doctor about all of your medical conditions, including if you have liver or kidney problems.
The most common side effects of MYRBETRIQ include high blood pressure, pain or swelling of the nose or throat (nasopharyngitis), urinary tract infection, and headache.
For further information, please talk to your healthcare professional and see accompanying Patient Product Information and complete Prescribing Information for MYRBETRIQ® (mirabegron extended‑release tablets).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Use of Myrbetriq
MYRBETRIQ® (mirabegron extended-release tablets) is a prescription medicine for adults used to treat overactive bladder (OAB) with symptoms of urgency, frequency and leakage.
Do not take MYRBETRIQ if you are allergic to mirabegron or any ingredients in MYRBETRIQ.
MYRBETRIQ may cause your blood pressure to increase or make your blood pressure worse if you have a history of high blood pressure. You and your doctor should check your blood pressure while you are taking MYRBETRIQ. Call your doctor if you have increased blood pressure.
MYRBETRIQ may increase your chances of not being able to empty your bladder. Tell your doctor right away if you have trouble emptying your bladder or you have a weak urine stream. MYRBETRIQ may cause an allergic reaction with swelling of the face, lips, throat or tongue with or without difficulty breathing. Stop using MYRBETRIQ and go to the nearest hospital emergency room right away.
Tell your doctor about all the medicines you take including medications for overactive bladder or other medicines especially thioridazine (Mellaril™ and Mellaril-S™), flecainide (Tambocor®), propafenone (Rythmol®), digoxin (Lanoxin®) or solifenacin succinate (VESIcare®). MYRBETRIQ may affect the way other medicines work, and other medicines may affect how MYRBETRIQ works.
Before taking MYRBETRIQ, tell your doctor about all of your medical conditions, including if you have liver or kidney problems.
The most common side effects of MYRBETRIQ include high blood pressure, pain or swelling of the nose or throat (nasopharyngitis), urinary tract infection, and headache.
For further information, please talk to your healthcare professional and see accompanying Patient Product Information and complete Prescribing Information for MYRBETRIQ® (mirabegron extended‑release tablets).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Getting Started
OAB Resources